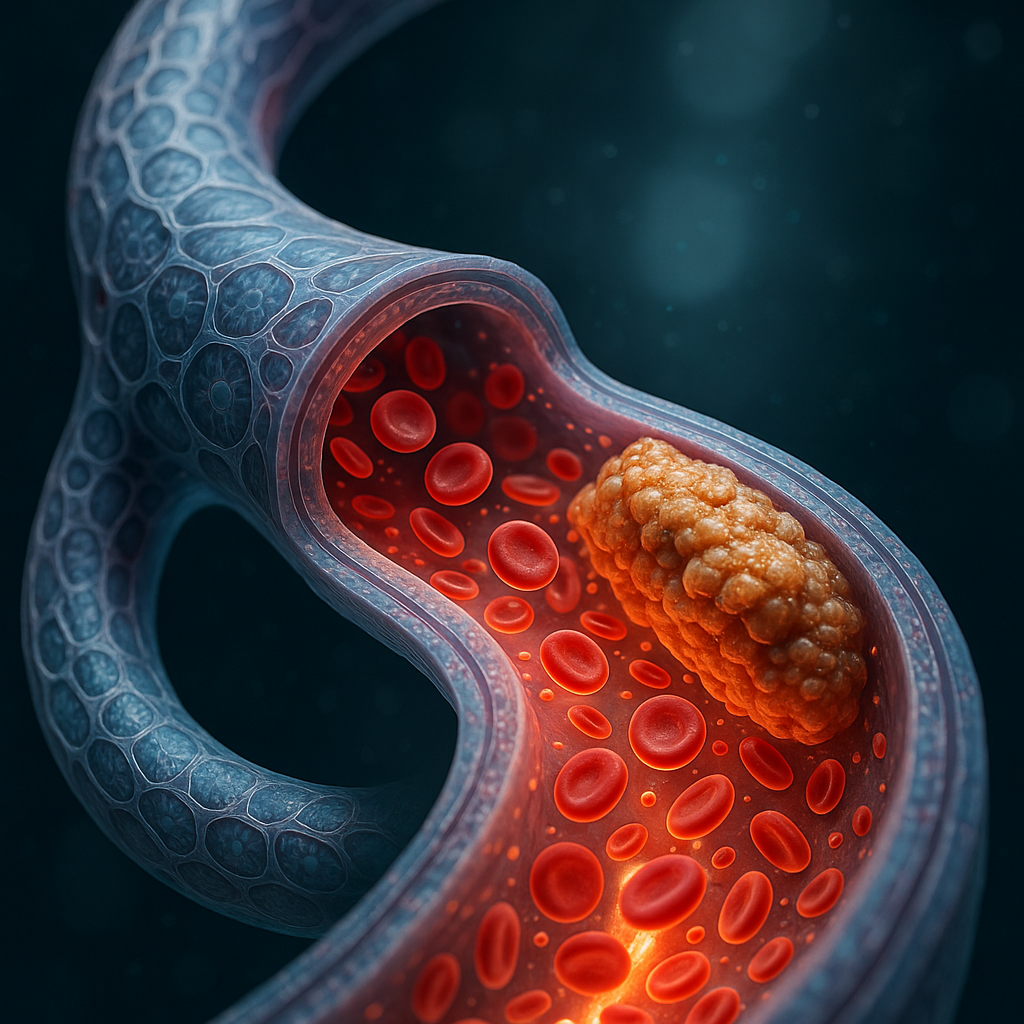
Recent developments in bioprinting have yielded a novel brain vessel model that mimics atherosclerotic blood flow, representing a significant leap forward in drug testing and biomedical research. Reported by 3D Printing Industry, this breakthrough utilizes bioprinting to create brain vessel structures that replicate the complex flow dynamics seen in atherosclerosis, a major cause of stroke and cardiovascular disease.
What Happened
Researchers have engineered a bioprinted brain vessel model that accurately replicates the flow conditions characteristic of atherosclerotic vessels. This model enables more realistic in vitro drug testing environments, allowing pharmaceutical developers to evaluate drug efficacy and safety under conditions that closely mimic human pathology. The model integrates living cells within a 3D-printed scaffold, producing a bio-embedded material that demonstrates both structural and functional fidelity to diseased brain vasculature.
Why It Matters
Atherosclerosis in cerebral vessels is a leading contributor to strokes and related neurological deficits. Traditional drug testing often relies on simplified models or animal studies that fail to capture the nuanced biological and mechanical environment of human brain arteries affected by plaque buildup. Bioprinted vessel models that replicate these conditions provide a more predictive platform for assessing drug performance, potentially reducing costly late-stage clinical failures and accelerating therapeutic development.
Moreover, this innovation exemplifies the broader potential of bio-embedded materials—living tissues integrated within engineered constructs—that could transform personalized medicine, regenerative therapies, and disease modeling.
Technical Context
The bioprinted brain vessel model leverages advances in hydrogel-based bioinks and high-resolution extrusion bioprinting. The bioinks are formulated to support endothelial and smooth muscle cell viability while maintaining mechanical properties that simulate arterial compliance and stiffness variations seen in atherosclerosis.
Key technical achievements include reproducing plaque-induced flow disturbances such as turbulent and oscillatory shear stress, which are critical to understanding disease progression and drug interaction. The model likely incorporates microfluidic control to simulate pulsatile blood flow, although specific details on flow rates and cell types used remain undisclosed.
This approach integrates multidisciplinary expertise from materials science, cell biology, and fluid dynamics, highlighting the complexity of creating functional bio-embedded constructs that faithfully replicate human pathophysiology.
Near-Term Prediction Model
The technology currently resides at the Pilot stage of maturity, with active research and validation underway. Within the next 12 to 24 months, we can expect incremental improvements in model complexity, including incorporation of immune cells and extracellular matrix components to better mimic the inflammatory aspects of atherosclerosis.
Pharmaceutical companies and academic labs will likely adopt these models for preclinical drug screening, potentially leading to commercial partnerships with bioprinting firms. Regulatory acceptance remains a hurdle, but growing evidence of improved predictive accuracy may accelerate validation pathways.
What to Watch
- Publication of validation studies comparing drug responses in bioprinted models versus animal and clinical data.
- Integration of multi-cellular components and vascular niche elements to enhance physiological relevance.
- Development of standardized protocols for reproducibility and scalability of bioprinted vessel models.
- Emergence of commercial bio-embedded platforms offering customizable disease models for pharmaceutical use.
- Regulatory agency guidance on acceptance criteria for bioprinted tissue models in drug approval processes.
While this brain vessel model marks a promising advance, details on long-term viability, scalability, and cost-efficiency of the bioprinting process remain to be clarified. Continued interdisciplinary collaboration will be essential to unlock the full potential of bio-embedded materials in translational medicine.