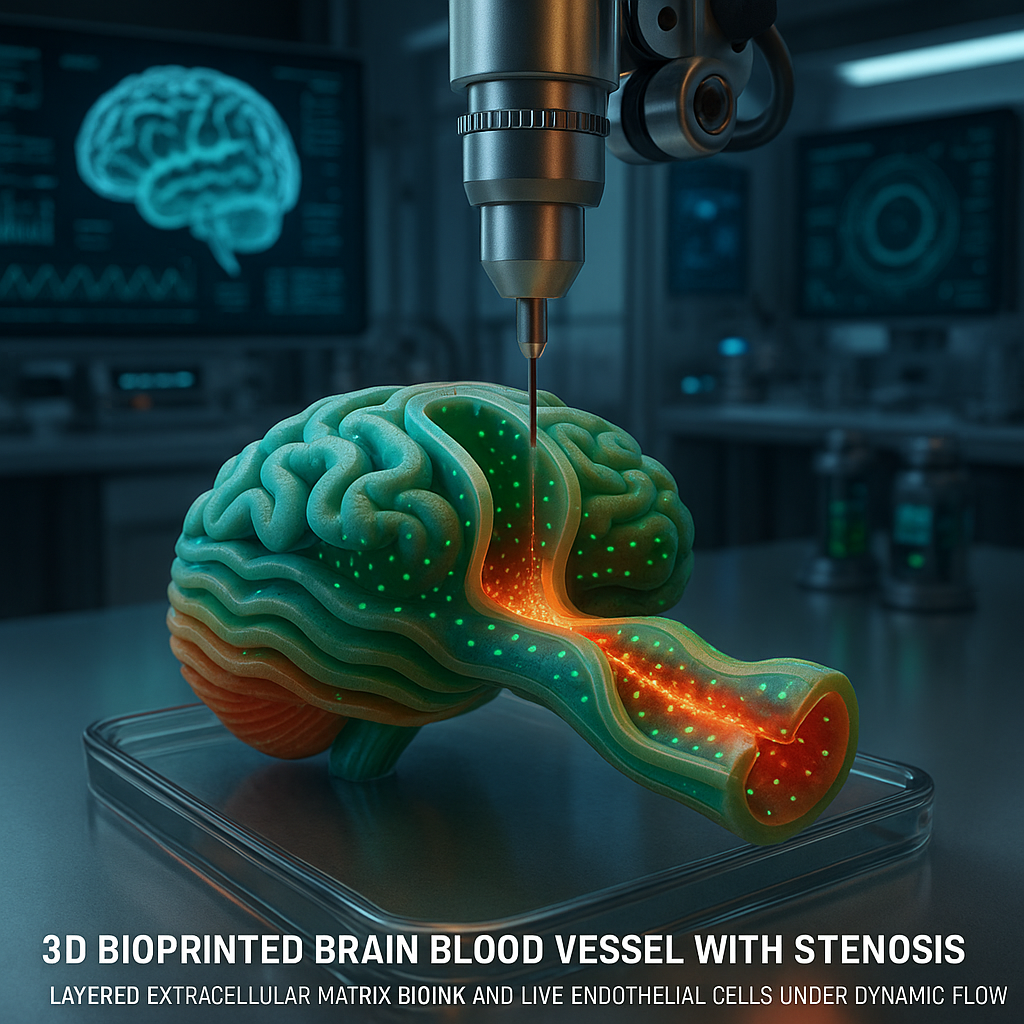
What Happened
Researchers have developed a novel bio-embedded 3D coaxial bioprinting technique to fabricate stenotic brain vessels using a mechanically enhanced extracellular matrix (ECM) bioink. This breakthrough, detailed in a recent Advanced Functional Materials article by Park et al. published via Wiley, enables the in vitro study of how hemodynamic forces influence endothelial cell responses within narrowed cerebral vessels.
Why It Matters
Brain vessel stenosis, the narrowing of blood vessels in the brain, is a critical factor in stroke and other cerebrovascular diseases. Traditional models have struggled to replicate the complex geometry and mechanical environment of stenotic vessels, limiting understanding of endothelial cell behavior under physiological flow conditions. This new bioprinting approach integrates bio-embedded materials with coaxial printing to produce structurally and mechanically relevant brain vessel models, offering unprecedented fidelity for biomedical research. It opens pathways for advanced disease modeling, drug screening, and potentially personalized medicine targeting vascular pathologies.
Technical Context
Coaxial bioprinting involves extruding multiple bioinks simultaneously through concentric nozzles, allowing creation of hollow tubular structures mimicking blood vessels. The innovation here lies in the mechanical enhancement of the ECM-based bioink, which traditionally suffers from low stiffness and poor mechanical integrity. By reinforcing the ECM bioink, the researchers achieved vessels capable of withstanding physiological flow and reproducing stenotic geometries. The bio-embedded nature means living cells are incorporated directly within the bioink matrix during printing, maintaining viability and function. This contrasts with post-print cell seeding methods and allows more accurate modeling of endothelial responses to shear stress and pressure changes induced by stenosis.
However, details on the specific mechanical reinforcement strategies, cell types used, and long-term stability of the printed vessels remain limited in the public domain. Further studies are likely needed to validate these models against in vivo conditions fully.
Near-Term Prediction Model
Given the current research stage, this technology is in the R&D phase with promising pilot demonstrations. Within 12 to 24 months, we anticipate pilot-scale adoption in academic and pharmaceutical research labs focused on cerebrovascular disease. The impact score is moderate to high (around 75/100) due to the potential to transform disease modeling and drug testing workflows. Confidence is medium (65/100) because while the concept is sound, translation to robust, reproducible models and integration into existing research pipelines require further validation.
What to Watch
- Publication of detailed protocols and mechanical characterization data for the enhanced ECM bioink.
- Demonstrations of long-term endothelial cell viability and functional responses under dynamic flow conditions.
- Comparative studies validating the printed stenotic vessels against animal models or clinical data.
- Expansion of bio-embedded coaxial printing to other vascular geometries and tissues.
- Commercialization efforts or collaborations between academic groups and biotech companies focusing on vascular disease modeling.
In summary, this advancement represents a significant step forward in bio-embedded 3D printing technology for brain vessel research. By combining mechanical enhancement with coaxial bioprinting, it addresses longstanding challenges in replicating complex vascular pathologies in vitro. Continued development and validation will determine its ultimate impact on biomedical research and therapeutic development.